INDICATIONS:
Venetoclax in combination with Rituximab is indicated for the treatment of adult patients with chronic lymphocytic leukaemia (CLL) who have received at least one prior therapy. Venetoclax monotherapy is indicated for the treatment of CLL: • In the presence of 17p deletion or TP53 mutation in adult patients who are unsuitable for or have failed a B-cell receptor pathway inhibitor, or • In the absence of 17p deletion or TP53 mutation in adult patients who have failed both chemoimmunotherapy and a B-cell receptor pathway inhibitor.
DOSAGE & ADMINISTRATION The starting dose is 20 mg of Venetoclax once daily for 7 days. The dose must be gradually increased over a period of 5 weeks up to the daily dose of 400 mg as shown in Table 1.
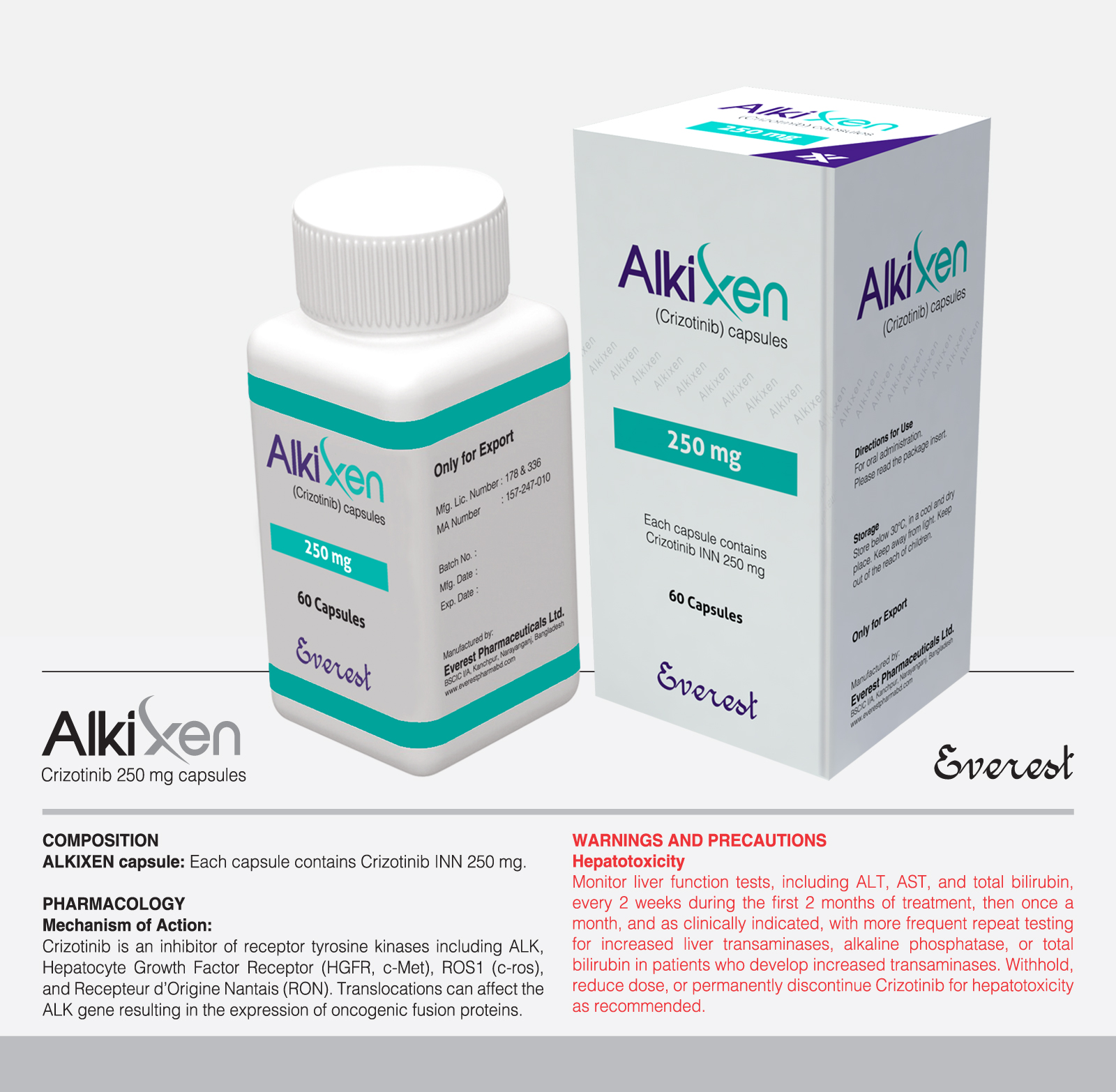
Tyrosine kinase inhibitor

Tyrosine kinase inhibitor

Tyrosine kinase inhibitor
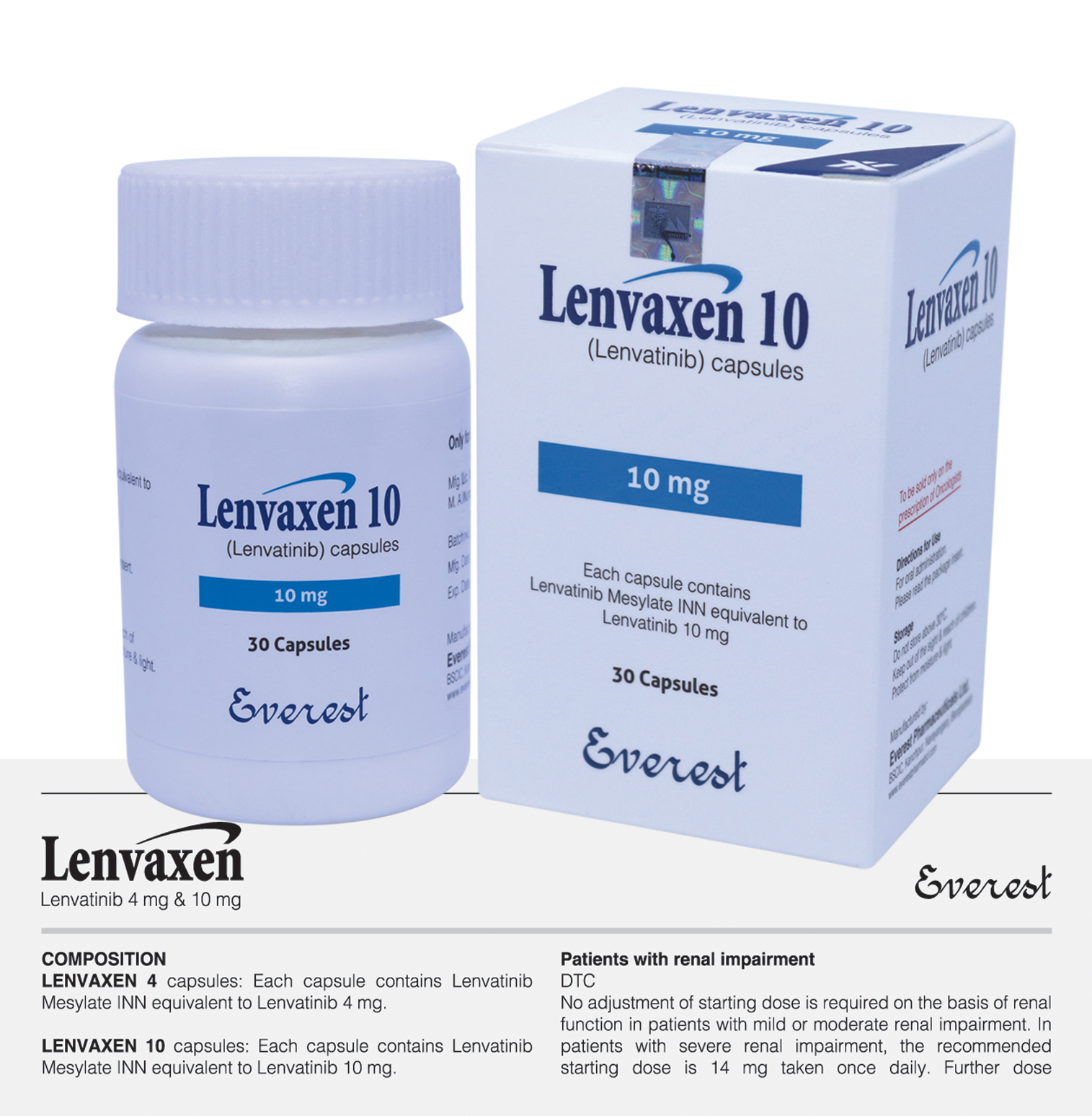
Tyrosine kinase inhibitor
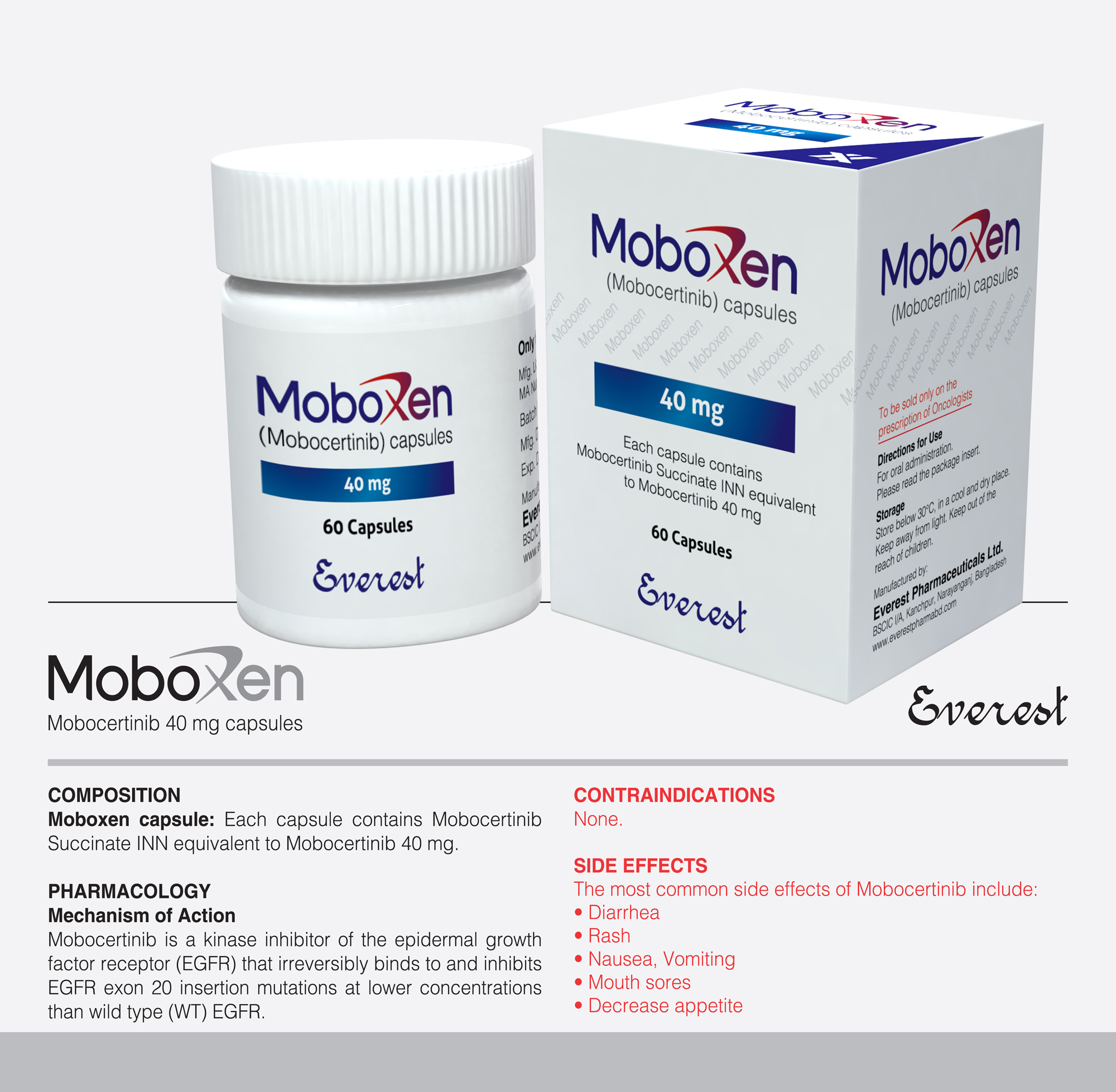
Tyrosine kinase inhibitor

polymerase (PARP) inhibitors
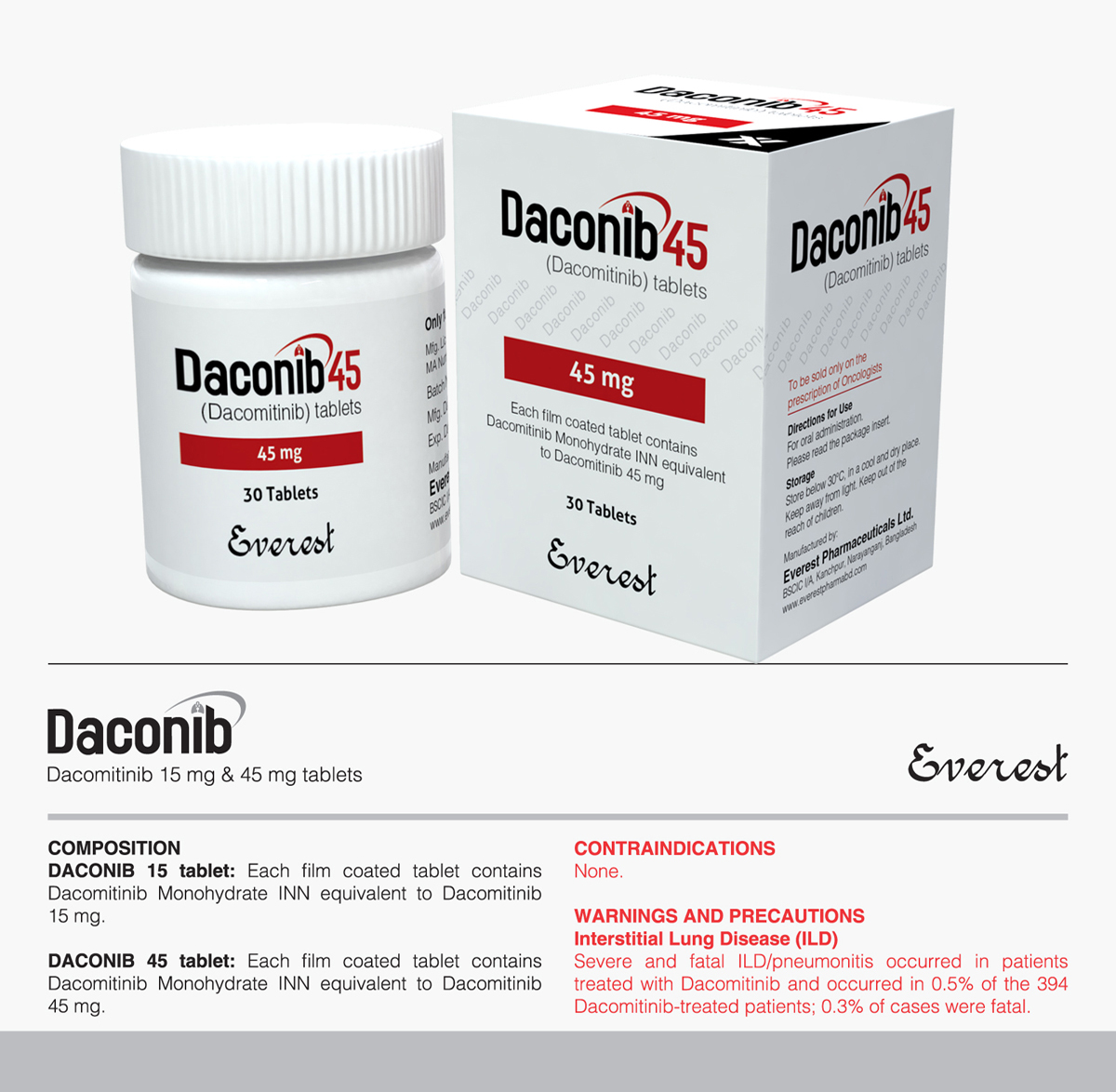
Tyrosin Kinase Inhibitor (TKI)
B-cell lymphoma-2 (BCL-2) inhibitors
Tyrosin Kinase Inhibitor (TKI)

Mezoxia